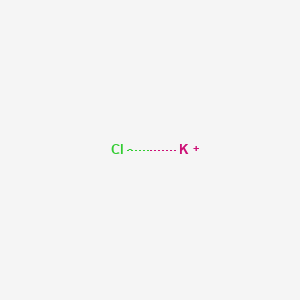Kcl Aqueous Solution

An aqueous solution of kcl would be.
Kcl aqueous solution. What is the mole fraction of kcl in the aqueous solution. Redox and the conversion to potassium metal edit although potassium is more electropositive than sodium kcl can be reduced to the metal by reaction with metallic sodium at 850 c because the more volatile potassium can be removed by distillation see le chatelier s principle. See also density of aqueous solutions of inorganic chlorides inorganic sodium salts some other inorganic substances organic acids and organic. Korean journal of chemical engineering 2017 34 4 977 986.
Prediction of the self diffusion coefficients in aqueous kcl solution using molecular dynamics. The mass fraction eq w i eq is a unit of composition based on the mass. Molarity moles of solute liter of solution values are tabulated below the figures. Molality moles of solute kg of water mol liter.
Acidic neutral or basic. Get 1 1 help now from expert chemistry tutors. In aqueous solution it is essentially fully ionized into solvated k and cl ions. Similar to previously reported data for nacl the spectra of both salts are well fitted by a cole cole equation although a double debye model is.
Be aware of the concentration units in the figures. For example when an aqueous solution of silver nitrate agno3 is added to the aqueous solution of sodium chloride nacl a white precipitate of silver chloride agcl is formed that is indicated by the following chemical reaction. Best answer 100 4 ratings previous question next question get more help from chegg. The attraction of hydrogen ion by chloride ion will release oh ion cl h 2 o hcl oh the production of oh will make the solution basic ph.
When the aqueous solution of kcl is electrolyzed then it will dissociate into potassium and chlorine ionskcl k cl the chloride ion produced will attract the hydrogen ion from water molecule while potassium will not hydrolyze. Ionic compounds dissociate into ions when dissolved in water aqueous solution. 1 answer anor277 jul 13 2018. In an aqueous solution of potassium chloride the solute is.
A comparative study of two force fields. Results of a dielectric relaxation study of the aqueous solutions of kcl and cscl at 25 c are reported using measurements with a vector network analyzer 0 2 ν ghz 20 and with waveguide interferometers 27 ν ghz 89. Mass and mole fraction. An aqueous solution contains 32 7 kci weight weight.